¿Qué tipo de flores escoger para un difunto? Novedosos tipos de flores para difuntos y funerales A la llegada del Día de
More¡Revolución Hogareña a Bajo Costo! Descubre la Casa Prefabricada y Móvil que es Furor en Amazon 🏠✨ Descubre la Casa Prefabricada y
MoreTransforma tu refugio: Ideas inspiradoras, como decorar tu dormitorio. Unas cuantas Ideas Geniales 🛏️✨ Buenos días, soy Johnny Zuri y HOY quiero
MoreHornos Bolero Hexa: Innovación y Eficiencia en tu Cocina – Revoluciona tu Cocina con Tecnología de Vanguardia. La cocina moderna demanda electrodomésticos
MoreSistemas de Tuberías Innovadores: Revolucionando el Futuro del Hogar 🚀💧 Tipos y Avances Tecnológicos 🏡🌍. En la vanguardia de la innovación y
MoreLas floristerías del futuro ¿Están las floristerías preparadas para las tendencias futuristas y vintage? En la era de la digitalización, la conexión
MoreDescubriendo las Maravillas de Floret Originals. Floret Originals: Un Viaje por el Mundo de las Semillas Únicas. Buenos días, soy Johnny Zuri
MoreMagia Floral y decoración con flores: Cómo Transformar tu Evento con una Decoración de Flores Exquisita. La decoración del 2024 nos invita
MoreDecoración Futurista en el Mundo: Un Vistazo al Futuro del Diseño de Interiores. El Impacto Visual de la Arquitectura Futurista En el
MoreLos mocasines son zapatos cómodos, versátiles y de diseño atemporal que han sido apreciados por diferentes culturas a lo largo de la
MoreLa iluminación adecuada en la habitación de un niño no solo es fundamental para su bienestar y desarrollo, sino que también puede
MoreSan Valentín es una fecha en la que las parejas aprovechan para demostrar todo su amor y cariño. Entre las diversas opciones
MoreRetro Elegancia en la Cocina: Tostadoras con Estilo La Fusión del Pasado y el Futuro en Electrodomésticos. El auge de lo retrofuturista
MoreHogares Smart: La Revolución del Confort y la Tecnología en el Hogar 🏡🤖 Innovación y Futuro en el Confort Doméstico 🚀 El
MoreSumérgete en el Lujo: Innovaciones en Piscinas Prefabricadas para Espacios Compactos. Innovación y Estilo. Innovación y Estilo en Espacios Reducidos El mundo
MoreRenovación de Cocinas en Zurilandia: Tradición y Tecnología en el Corazón del Hogar Transforma tu Cocina en Zurilandia | Ideas Innovadoras y
MoreLos Mejores Ventiladores de Mesa Pequeños y Silenciosos para tu Espacio de Trabajo Ventilador de Mesa Pequeño y Silencioso: Guía de Compra.
MoreDecore Cuenca: Trucos para transformar tu hogar sin romper tu contrato de alquiler… Conozca algunos trucos de decoración sin infringir su contrato
MoreDesentrañando el Encanto de las Bodas Temáticas y Vintage: Una Revolución Floral y Vintage🌸🎩 La magia que envuelve las Bodas Temáticas ha
MoreDescubre las Lámparas de Pie Nordicas que Transformarán tu Espacio. 🛋️ ¡Ilumina con estilo! 🌟 Descubrir el encanto de una lampara de
More5 cosas que Debes Saber sobre Sujeciones de Hormigón Prefabricado. 🌟 ¿El Futuro de la Construcción? En el mundo de la construcción,
More¿Vuelve Prisunic a Revolucionar la Decoración Vintage? 🌟 Prisunic: El regreso más esperado en el mundo de la “DECORACIÓN” 🌟 ¿Qué sorpresas
More¿El Estilo Japandi es el Futuro del Diseño de Interiores? 🌸🏯 Descubre el futuro del diseño de interiores con el estilo Japandi
More¡Descubre los Azulejos para Piscinas Modernas que Revolucionarán tu Verano! 🌊🌞 Descubre las tendencias. En el mundo de las piscinas, cada detalle
MoreMadera Curvada al Vapor: El Secreto Detrás de la Silla PK15 de Poul Kjærholm 🌳🛋️ Descubre cómo la madera curvada al vapor
MoreArquitectos para restaurantes: La revolución futurista y vintage del diseño de espacios gastronómicos 🍽️🏗️ Arquitectos para restaurantes: Cómo el diseño arquitectónico está
MoreBricolaje y Decoración. 🎨🖌️🎨 Pinturas y Brochas para Decoración Vintage y Flores en Cuenca 🌸 En el mundo del bricolaje y la
MoreTipos de piscinas modernas: Descubre el futuro del lujo y la decoración. El Lujo Futurista en Agua… Tipos de Piscinas Modernas: El
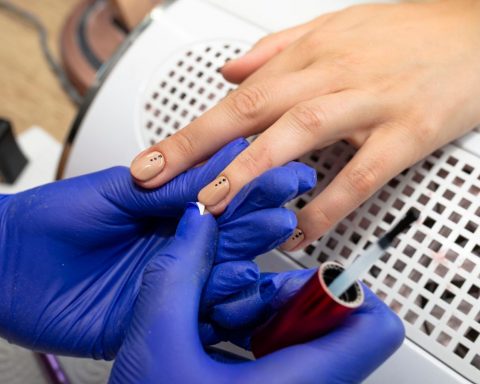
MoreLas uñas semipermanentes de Semilac son la solución perfecta para quienes desean lucir unas uñas impecables sin tener que preocuparse por los retoques constantes.
More¿Cómo el diseño futurista está revolucionando las casas experimentales? De la Innovación al Diseño Futurista. En la vasta intersección entre la tecnología
MoreVariedades de uva de mesa sin semilla. ¿Tu propio vino con la mejor uva? ¡Descubre Viveros Barber! En el universo vitivinícola, una
More¿Cuál es el Mejor Calzado para Jardinería? ¿Un fabricante de calzado de seguridad para jardinería? El cuidado y embellecimiento de los espacios
More